Society: AGA
Attendees should attend this session to learn about practical examples of successful quality improvement projects for integration into their day to day work lives. This session will also provide guidance on how to develop a quality improvement project.
BACKGROUND: Exocrine pancreatic insufficiency (EPI) is common in chronic pancreatitis (CP), pancreatic cancer (PDAC), and post pancreatic resection. Only about 1/3 of these patients are prescribed pancreatic enzyme replacement therapy (PERT), often at an inadequate dose. There is increasing evidence that this leads to increased morbidity and mortality. The aim of this study was to identify practice patterns of EPI treatment , develop and implement an EPIC-based best practice alert (BPA) and smart order set with goal of improving management of EPI.
METHODS: All patients with ICD-10 codes of EPI, CP, and PDAC or CPT code for pancreatic resection, who were seen in either an outpatient or inpatient setting at a tertiary care center from Feb-2018 to June-2022 were included. Appropriate use of PERT was defined as > 40,000 USP units of lipase with each meal. The initial retrospective analysis was conducted prior to implementation of the BPA and smart set from feb-2018 to feb-2020. The BPA and smart set were implemented on Feb-2020 and another analysis was done on patients from feb-2020 to June 2022.
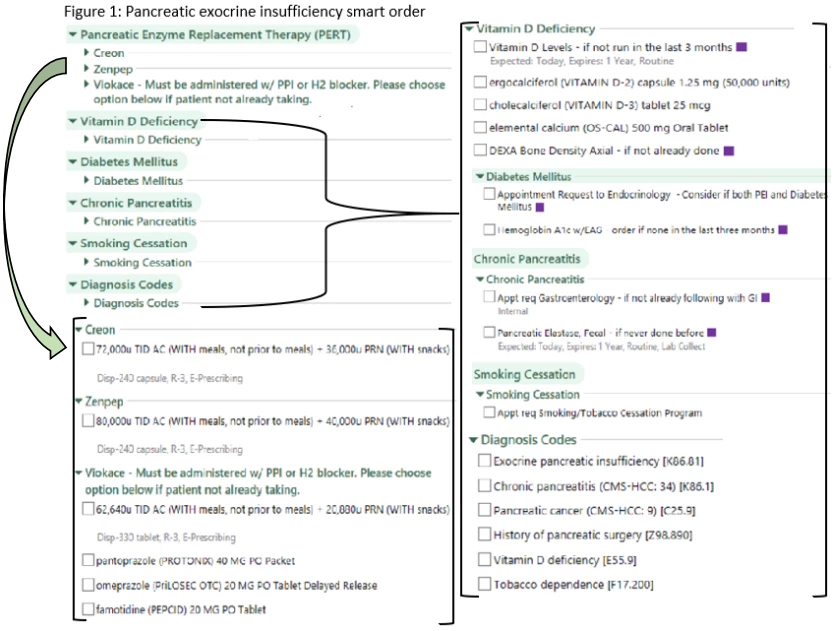
RESULTS: The baseline analysis identified 1,464 patients carrying an ICD-10 code for CP, EPI, PDAC or a CPT code for pancreatic resection whose diagnosis was confirmed by manual chart review . Overall, 837 (57.2%) patients were prescribed PERT. Of those prescribed enzymes, 518 (61.9%) were on a less than minimum therapeutic dose. Overall, 299 (20.4%) patients had a pancreatic elastase checked, 453 (30.9%) had a vitamin D level measured, 156 (10%) had a DEXA ordered, and 801 (54.7%) had a hemoglobin A1c level checked. An order set was designed, with an associated BPA tied to any of these diagnoses or to any order for PERT (Figure 1).The BPA and order set were incorporated into our EPIC system and are now in active use.
A statistically significant increase in the proportion of patients on minimum therapeutic dose of PERT from 61.9 to 72.9% (P=<0.001) was observed. Ordering of pancreatic elastase, A1c, vitamin D, and DEXA increased from 20.4 to 29.9% (P<0.001), 54.7 to 62.1% (P=0.001), 30.9 to 48.1% (P<0.001) and 10 to 18% (P<0.001), respectively after initiation of BPA and smart set. An increase in vitamin D supplementation from 25.5 to 33.4% (P<0.001) and a decrease in the proportion of patients with unknown metabolic bone disease status from 86.8 to 76.8% (P<0.001) was observed.
CONCLUSIONS: The implementation of a BPA and smart set for the management of exocrine pancreatic insufficiency was associated with an improvement in management and monitoring noted by an increase in proportion of patients on minimum therapeutic dose of enzyme as well as by an increase in ordering of pancreatic elastase, A1c, vit D, DEXA scan to monitor disease related complicatons
Background:
Many patients with symptoms of gastroesophageal reflux disease (GERD) despite proton pump inhibitor (PPI) use undergo ambulatory reflux testing, which is ideally performed off therapy in the absence of a prior GERD diagnosis according to Porto Consensus recommendations. However, we previously identified substantial variation in reflux testing patterns among such patients at our institution, with a majority undergoing multichannel intraluminal impedance pH (MII-pH) testing on PPI therapy without previous objective evidence of GERD. We aimed to assess the impact of a novel clinical decision support tool (CDST) on adherence to best practices for GERD testing.
Methods:
We used Define, Measure, Analyze, Improve, and Control (DMAIC) methodology to identify factors associated with MII-pH ordering discordant from best practices, with clinician knowledge gaps and lack of standardization in ordering options as contributors (Figure 1). These results motivated the development of a CDST within the electronic health record (EHR), which was updated to default MII-pH testing off therapy whereas testing on PPI requests designating the prior GERD history. Clinicians may still select testing on PPI by manually entering a justification. We assessed the impact of this intervention on consecutive patients referred for ambulatory MII-pH testing from the inception of the CDST on 1/1/2022 through 10/31/2022. Patients with prior foregut surgery, undergoing testing for lung transplantation, or referred from outside centers without EHR access were excluded. Appropriate statistical tests were used to compare the proportion of patients undergoing ambulatory reflux testing on therapy and concordant with best practices before and after implementation of the CDST.
Results:
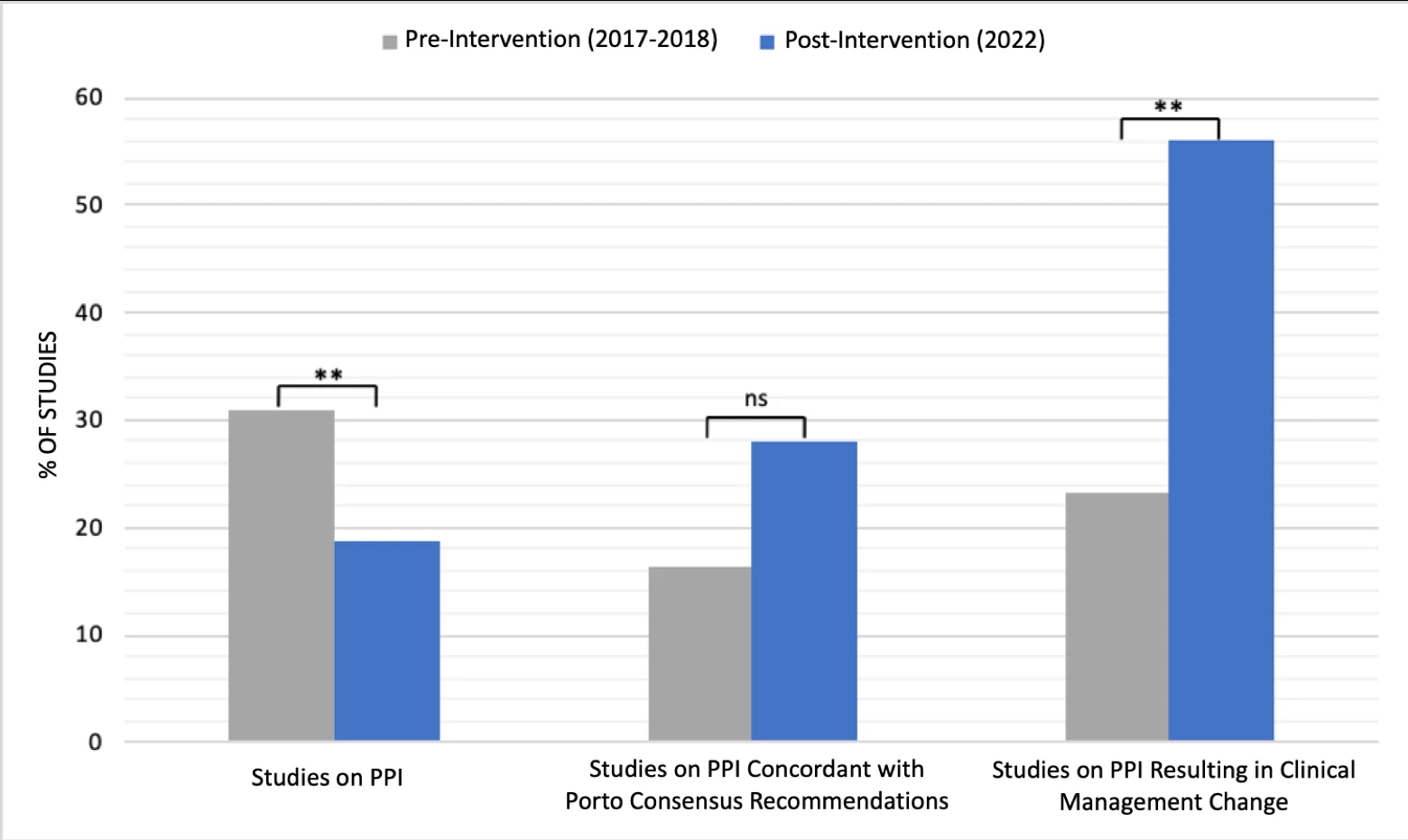
There were 567 MII-pH studies evaluated, including 433 studies pre-intervention and 134 post-intervention. Our center performed significantly fewer studies on PPI after the CDST than before (n=25/134, 18.7% vs n=134/433, 30.9%; p<0.01). Of those studies performed on PPI, concordance with best practices increased from 16.4% (n=22/134) to 28.0% (n=7/25) following the intervention. Additionally, the CDST resulted in significantly more clinical management changes after implementation among those tested on therapy (n=31/134, 23.1% vs n=14/25, 56.0%; p<0.01) (Figure 2).
Conclusions:
To address the observed variation in care and MII-pH test ordering that frequently contrasted with best practices, we used DMAIC methodology to develop a CDST. After our intervention, the number of MII-pH studies ordered on PPI was significantly reduced while ordering concordant with the Porto Consensus recommendations and changes in clinical management increased. Our results show a CDST can guide appropriate ambulatory reflux testing protocols and meaningfully impact the clinical management of patients with GERD symptoms.
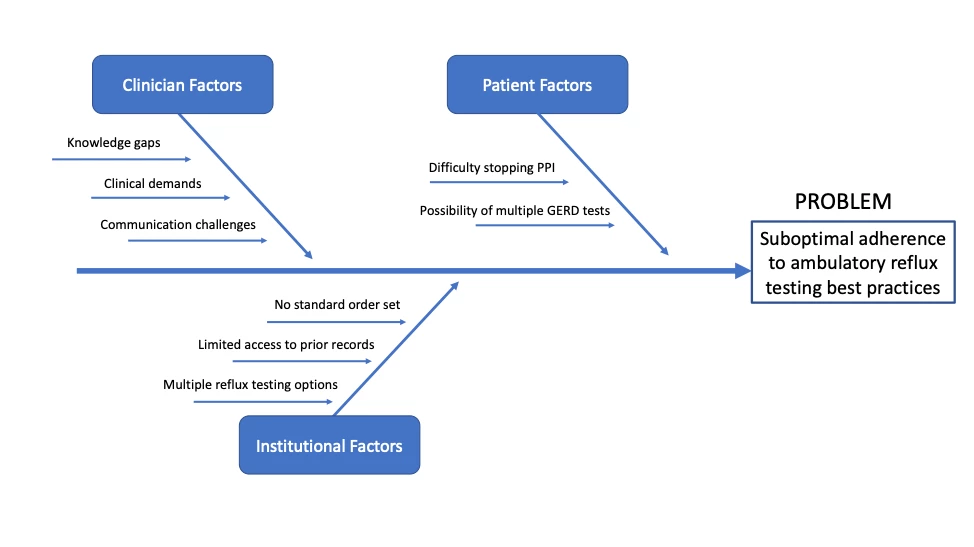
Figure 1. Ambulatory Reflux Testing Root-Cause Analysis. PPI, proton pump inhibitor. EHR, electronic health record.
Figure 2. Impact of Clinical Decision Support Tool. PPI, proton pump inhibitor. %, percentage. NS, not significant. ** indicates p<0.01.