The accreditors of this session require that you periodically check in to verify that you are still attentive. Please click the button below to indicate that you are.
835
EARLY LIFE ADVERSITY DISRUPTS SEXUAL DIMORPHISM OF ENTERIC GLIA
Date
May 8, 2023
Explore related products in the following collection:
Society: AGA
Background
Electrogastrography (EGG) non-invasively evaluates gastric motility but is widely viewed as lacking clinical utility. Gastric Alimetry® is a new diagnostic test that combines high-resolution body surface gastric mapping (BSGM) with validated symptom profiling, with the goal of overcoming numerous technical limitations of the EGG. This study directly compared EGG and BSGM to define performance differences in spectral analysis.
Methods
Comparisons between Gastric Alimetry BSGM and EGG were conducted by protocolized evaluation of 178 subjects (110 controls; 68 nausea and vomiting (NVS) and/or type 1 diabetes (T1D)). Data collection was identical. Comparisons followed standard methodologies for each test, with statistical evaluations for group-level differences, symptom correlations, and patient-level classifications. Four spectral metrics were computed for BSGM tests (Gastric Alimetry Rhythm IndexTM (GA-RI), Principal Gastric Frequency (PGF), BMI-Adjusted Amplitude, and Fed:Fasted Amplitude Ratio)1 and EGG tests (% time normal frequency, dominant frequency, amplitude, and amplitude ratio)2. Patient-level classifications were determined by a blinded consensus panel reference standard3 and automatedly, using published reference values as cutoffs for EGG and BSGM metrics2,4.
Results
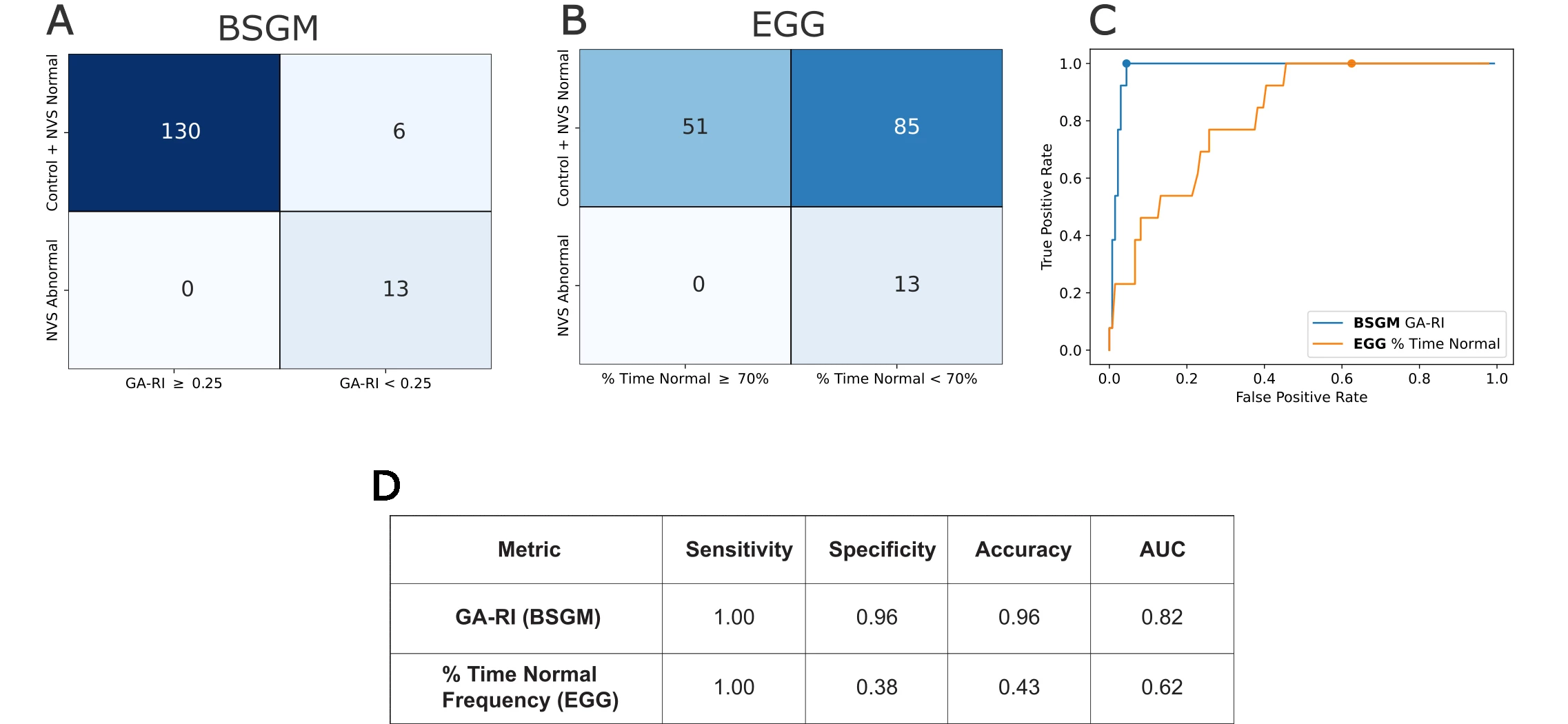
Group-level: BSGM showed tighter frequency ranges vs EGG in controls (median 3.04 cpm (IQR 2.90-3.18) vs 2.88 (1.50-3.12); p<0.0001). Both tests detected rhythm instability in NVS (p<0.001) and T1D (p<0.05), but EGG showed opposite frequency effects in T1D (2.50 vs controls 2.88; p=0.28) to BSGM (3.15 vs 3.04; p=0.0004). Symptom correlations: GA-RI correlated with nausea, pain, bloating, and total symptom burden; PGF deviation with excessive fullness, pain, bloating; % time in normal frequency correlated with bloating (p<0.05). Patient-level: EGG sensitivity was 1.0, specificity 0.38; BSGM sensitivity 1.0, specificity 0.96 (Figure 1).
Conclusions and Inferences
EGG detected group-level differences in patients, but lacked symptom correlations and showed poor accuracy at patient-level classification, explaining EGG’s weak clinical utility. BSGM demonstrated substantial performance improvements over EGG across all domains.
References
1. Schamberg G et al.. Neurogastroenterol Motil. Published online November 21, 2022:e14491.
2. Yin J, Chen JDZ. Journal of Neurogastroenterology and Motility. 2013;19(1):5-17.
3. Gharibans AA et al. Sci Transl Med. 2022;14(663):eabq3544.
4. Varghese C et al. Am J Gastroenterol; In Press [doi:10.1101/2022.07.25.22278036]
Figure 1: Individual classifications using rhythmic stability metrics and reference labels. (A) Confusion matrix for GA-RI ≥ 0.25. (B) Confusion matrix for % time normal frequency ≥ 70%. (C) ROC curves with the location on the curve associated (published thresholds indicated by a dot). (D) Quantitative evaluation.
Background
Electrogastrography (EGG) non-invasively evaluates gastric motility but is widely viewed as lacking clinical utility. Gastric Alimetry® is a new diagnostic test that combines high-resolution body surface gastric mapping (BSGM) with validated symptom profiling, with the goal of overcoming numerous technical limitations of the EGG. This study directly compared EGG and BSGM to define performance differences in spectral analysis.
Methods
Comparisons between Gastric Alimetry BSGM and EGG were conducted by protocolized evaluation of 178 subjects (110 controls; 68 nausea and vomiting (NVS) and/or type 1 diabetes (T1D)). Data collection was identical. Comparisons followed standard methodologies for each test, with statistical evaluations for group-level differences, symptom correlations, and patient-level classifications. Four spectral metrics were computed for BSGM tests (Gastric Alimetry Rhythm IndexTM (GA-RI), Principal Gastric Frequency (PGF), BMI-Adjusted Amplitude, and Fed:Fasted Amplitude Ratio)1 and EGG tests (% time normal frequency, dominant frequency, amplitude, and amplitude ratio)2. Patient-level classifications were determined by a blinded consensus panel reference standard3 and automatedly, using published reference values as cutoffs for EGG and BSGM metrics2,4.
Results
Group-level: BSGM showed tighter frequency ranges vs EGG in controls (median 3.04 cpm (IQR 2.90-3.18) vs 2.88 (1.50-3.12); p<0.0001). Both tests detected rhythm instability in NVS (p<0.001) and T1D (p<0.05), but EGG showed opposite frequency effects in T1D (2.50 vs controls 2.88; p=0.28) to BSGM (3.15 vs 3.04; p=0.0004). Symptom correlations: GA-RI correlated with nausea, pain, bloating, and total symptom burden; PGF deviation with excessive fullness, pain, bloating; % time in normal frequency correlated with bloating (p<0.05). Patient-level: EGG sensitivity was 1.0, specificity 0.38; BSGM sensitivity 1.0, specificity 0.96 (Figure 1).
Conclusions and Inferences
EGG detected group-level differences in patients, but lacked symptom correlations and showed poor accuracy at patient-level classification, explaining EGG’s weak clinical utility. BSGM demonstrated substantial performance improvements over EGG across all domains.
References
1. Schamberg G et al.. Neurogastroenterol Motil. Published online November 21, 2022:e14491.
2. Yin J, Chen JDZ. Journal of Neurogastroenterology and Motility. 2013;19(1):5-17.
3. Gharibans AA et al. Sci Transl Med. 2022;14(663):eabq3544.
4. Varghese C et al. Am J Gastroenterol; In Press [doi:10.1101/2022.07.25.22278036]
Figure 1: Individual classifications using rhythmic stability metrics and reference labels. (A) Confusion matrix for GA-RI ≥ 0.25. (B) Confusion matrix for % time normal frequency ≥ 70%. (C) ROC curves with the location on the curve associated (published thresholds indicated by a dot). (D) Quantitative evaluation.

Individual classifications using rhythmic stability metrics and reference labels. (A) Confusion matrix for GA-RI ≥ 0.25. (B) Confusion matrix for % time normal frequency ≥ 70%. (C) ROC curves with the location on the curve associated (published thresholds indicated by a dot). (D) Quantitative evaluation.
Purpose: Enteric neuropathies result from abnormalities of the enteric nervous system (ENS). The goal of this study was to determine the feasibility of isolating, expanding, and transplanting autologous enteric neural stem cells (ENSCs) to improve colonic function in a non-lethal mouse model of colonic aganglionosis.
Methods: Wnt1::Cre;R26iDTR (Wnt1-iDTR) mice at 2-3 months of age were used. ENSCs were isolated from a short segment of small intestine resected from Wnt1-iDTR mice in which focal colonic aganglionosis was created by injection of diphtheria toxin (DT) into the wall of the mid-colon. Autologous ENSCs were expanded in culture to form neurospheres, transduced with lentiviral GFP or channelrhodopsin-2 (ChR2) vector, and transplanted into the aganglionic segment 2 weeks following the first surgery (n=12). Immunohistochemistry, organ bath studies, and optogenetics were used to determine outcomes 1 to 8 weeks following cell transplantation. Results were compared to two groups: R26iDTR mice with DT injection (Control, n=8) and DT-induced aganglionosis (DT-AG, n=8) without cell transplantation using multiple t-test analysis.
Results: ENSCs, as neurospheres, were successfully obtained from mouse small intestine (mean 477 ± 123 neurospheres per cm of small intestine, n=12). Transplanted ENSCs were identified within the aganglionic colon based on GFP fluorescence. Immunohistochemical evaluation demonstrated extensive cell migration, nerve fiber projections, and differentiation into neurons and glial cells. Organ bath studies demonstrated that the lack of contraction of DT-induced aganglionic smooth muscle was rescued by ENSC transplantation (0.10 ± 0.09 gm in DT-AG vs 1.45 ± 0.23 gm in DT-AG + Cells, n = 4, p<0.001) 3 weeks following cell transplant. Interestingly, restoration of muscle contractility progressed over time. Contractile activity of colonic smooth muscle in mice with DT-induced aganglionosis was normalized by ENSC transplantation 8 weeks after surgery (3.11 ± 0.13 gm in Control vs 2.95 ± 0.39 gm in DT-AG + Cells, n = 4, p>0.05). Optogenetic analysis in mice receiving ENSCs expressing ChR2 confirmed functional neuromuscular signalling between transplanted autologous ENSC-derived neurons and the colonic smooth muscle.
Conclusions: Autologously derived ENSCs successfully engrafted within aganglionic gut in vivo, where neuroglial differentiation and functional integration with colonic smooth muscle occurred. By 8 weeks after cell transplant, normalization of colonic smooth muscle contractility was observed. This study, using a novel and non-lethal mouse model of colonic aganglionosis, demonstrates the potential of autologous ENSCs to improve functional outcomes in neurointestinal disease and lays the groundwork for clinical application of this regenerative cell-based strategy.
Methods: Wnt1::Cre;R26iDTR (Wnt1-iDTR) mice at 2-3 months of age were used. ENSCs were isolated from a short segment of small intestine resected from Wnt1-iDTR mice in which focal colonic aganglionosis was created by injection of diphtheria toxin (DT) into the wall of the mid-colon. Autologous ENSCs were expanded in culture to form neurospheres, transduced with lentiviral GFP or channelrhodopsin-2 (ChR2) vector, and transplanted into the aganglionic segment 2 weeks following the first surgery (n=12). Immunohistochemistry, organ bath studies, and optogenetics were used to determine outcomes 1 to 8 weeks following cell transplantation. Results were compared to two groups: R26iDTR mice with DT injection (Control, n=8) and DT-induced aganglionosis (DT-AG, n=8) without cell transplantation using multiple t-test analysis.
Results: ENSCs, as neurospheres, were successfully obtained from mouse small intestine (mean 477 ± 123 neurospheres per cm of small intestine, n=12). Transplanted ENSCs were identified within the aganglionic colon based on GFP fluorescence. Immunohistochemical evaluation demonstrated extensive cell migration, nerve fiber projections, and differentiation into neurons and glial cells. Organ bath studies demonstrated that the lack of contraction of DT-induced aganglionic smooth muscle was rescued by ENSC transplantation (0.10 ± 0.09 gm in DT-AG vs 1.45 ± 0.23 gm in DT-AG + Cells, n = 4, p<0.001) 3 weeks following cell transplant. Interestingly, restoration of muscle contractility progressed over time. Contractile activity of colonic smooth muscle in mice with DT-induced aganglionosis was normalized by ENSC transplantation 8 weeks after surgery (3.11 ± 0.13 gm in Control vs 2.95 ± 0.39 gm in DT-AG + Cells, n = 4, p>0.05). Optogenetic analysis in mice receiving ENSCs expressing ChR2 confirmed functional neuromuscular signalling between transplanted autologous ENSC-derived neurons and the colonic smooth muscle.
Conclusions: Autologously derived ENSCs successfully engrafted within aganglionic gut in vivo, where neuroglial differentiation and functional integration with colonic smooth muscle occurred. By 8 weeks after cell transplant, normalization of colonic smooth muscle contractility was observed. This study, using a novel and non-lethal mouse model of colonic aganglionosis, demonstrates the potential of autologous ENSCs to improve functional outcomes in neurointestinal disease and lays the groundwork for clinical application of this regenerative cell-based strategy.
[Background] Several studies have assessed the effect of cool temperature on gastrointestinal peristalsis. Transient receptor potential melastatin 8 (TRPM8) is a temperature-sensitive ion channel activated by mild cooling expressed in the gastrointestinal tract. We examined the antispasmodic effect of cool temperature on gastrointestinal peristalsis in a prospective, randomized, single-blind trial and based on the video imaging and intraluminal pressure of the proximal colon in TRPM8-deficient mice.
[Method] In the clinical trial, we randomly assigned a total of 86 patients scheduled to undergo esophagogastoroduodenoscopy to 2 groups (the mildly cool water [n=43] and room temperature (RT) [n=43] groups) and a total of 94 patients scheduled to undergo colonoscopy to 2 groups (the mildly cool water [n=47] and RT [n=47] groups). We used 20 mL of 15 °C water as mildly cool water. Both treatments were sprayed locally onto the gastric mucosa or colonic mucosa via an endoscope. The primary outcome was the proportion of subjects with improved peristalsis after treatment (Clinical trial registry website: Trial No. UMIN-CTR UMIN000030725). In the rodent colon model, we placed a 2- to 3-cm segment of the proximal colon in an organ bath (100 mL volume) and perfused the bath with Krebs solution (34-35 °C, 3.5 mL/min). We securely attached the oral and aboral ends of the proximal colon segment with string to the saline input and output ports, respectively. In the colon, we used a Mikro-Tip catheter pressure transducer (SPR-524; Millar Instruments, Houston, TX, USA) to monitor the intraluminal pressure (cmH2O). With a data acquisition and analysis system (MP100; BIOPAC Systems, Goleta, CA, USA), we evaluated the intraluminal pressure waves. We macroscopically observed motility through video (HDC-HS 100-K; Panasonic, Osaka, Japan). In the proximal colon of TRPM8-deficient mice and wild mice, we evaluated the intraluminal pressure and performed video imaging of the TRPM8-deficient mouse colon by administering cool water into the colonic lumen.
[Results] In the randomized controlled trial, after treatment, the proportion of subjects with no colonic peristalsis was significantly higher in the cool water group than in the RT group (44.7% vs. 23.4%; P < 0.05). Regarding gastric peristalsis, there were no significant differences between the cool water group and the RT group. In the rodent colon model, cool water was associated with a significant decrease in colonic peristalsis through its suppression of the peak frequency ratio (P < 0.05). Cool water-treated TRPM8-deficient mice did not show a reduction in colonic peristalsis compared with wild-type mice.
[Conclusion] We showed for the first time that cool temperature-dependent suppression of colonic peristalsis may be associated with TRPM8 activation.
[Method] In the clinical trial, we randomly assigned a total of 86 patients scheduled to undergo esophagogastoroduodenoscopy to 2 groups (the mildly cool water [n=43] and room temperature (RT) [n=43] groups) and a total of 94 patients scheduled to undergo colonoscopy to 2 groups (the mildly cool water [n=47] and RT [n=47] groups). We used 20 mL of 15 °C water as mildly cool water. Both treatments were sprayed locally onto the gastric mucosa or colonic mucosa via an endoscope. The primary outcome was the proportion of subjects with improved peristalsis after treatment (Clinical trial registry website: Trial No. UMIN-CTR UMIN000030725). In the rodent colon model, we placed a 2- to 3-cm segment of the proximal colon in an organ bath (100 mL volume) and perfused the bath with Krebs solution (34-35 °C, 3.5 mL/min). We securely attached the oral and aboral ends of the proximal colon segment with string to the saline input and output ports, respectively. In the colon, we used a Mikro-Tip catheter pressure transducer (SPR-524; Millar Instruments, Houston, TX, USA) to monitor the intraluminal pressure (cmH2O). With a data acquisition and analysis system (MP100; BIOPAC Systems, Goleta, CA, USA), we evaluated the intraluminal pressure waves. We macroscopically observed motility through video (HDC-HS 100-K; Panasonic, Osaka, Japan). In the proximal colon of TRPM8-deficient mice and wild mice, we evaluated the intraluminal pressure and performed video imaging of the TRPM8-deficient mouse colon by administering cool water into the colonic lumen.
[Results] In the randomized controlled trial, after treatment, the proportion of subjects with no colonic peristalsis was significantly higher in the cool water group than in the RT group (44.7% vs. 23.4%; P < 0.05). Regarding gastric peristalsis, there were no significant differences between the cool water group and the RT group. In the rodent colon model, cool water was associated with a significant decrease in colonic peristalsis through its suppression of the peak frequency ratio (P < 0.05). Cool water-treated TRPM8-deficient mice did not show a reduction in colonic peristalsis compared with wild-type mice.
[Conclusion] We showed for the first time that cool temperature-dependent suppression of colonic peristalsis may be associated with TRPM8 activation.
Irritable bowel syndrome (IBS) affects roughly 12% of humans and is more common in women than men. Peripheral and central neuroplasticity produce abdominal pain and altered intestinal function in IBS but the exact cause of neuroplasticity is not understood. Experiencing early life adversity increases susceptibility to developing IBS through an interplay between genes and the environment, yet how early life stress affects specific cell types and mechanisms in the enteric nervous system function remains unknown. Here, we tested the hypothesis that early life stress disrupts enteric nervous system function through genomic changes induced in enteric glia.
We used the neonatal-maternal separation (NMS) model as a psychological stressor, and studied glial-specific transcriptional signatures using Sox10CreERT2;RiboTag mice. Distal colons were collected at 16-20 weeks of age and RNA-seq of myenteric glial mRNA was performed using STAR and DESeq2. Specific transcripts of interest were localized in situ by RNAscope with co-immunolabeling to quantify cellular expression. Integrative physiological consequences were assessed by measuring visceral hypersensitivity and in vivo motility, and cellular physiological responses were studied using calcium imaging in Wnt1Cre;GCaMP5g-tdT/GfaphM3Dq mice, which express chemogenetic hM3Dq receptors in GFAP+ cells and GCaMP5g in neurons and glia.
In normal animals, more than 600 genes are differentially expressed between male and female enteric glia. Males that underwent NMS exhibited large shifts in glial genomic profiles with 469 genes upregulated, while subtler effects were observed in females with 124 genes downregulated. Interestingly, the gene expression patterns of male glia become more like those from females following NMS. In addition to the change in expression level, spatial distribution of target RNA, such as immune-related signaling gene Oasl2 (p<0.01) or Ifit1 (p<0.05), is affected by NMS. This remodeling of molecular architecture is associated with a higher excitability of enteric glia, demonstrated by an increased frequency of calcium responses after specific glial stimulation. Finally, we observed an increased visceral sensitivity in males after NMS (p<0.01), and a slower intestinal transit after glial specific activation in both sexes (p<0.05), which may be linked to the hyperexcitability of glia.
Together, these data show that early life adversity shifts the molecular architecture of enteric glia in a sex-specific manner, with a prominent effect in males where NMS promotes a ‘feminization’ of genomic signatures. This remodeling is associated with physiological consequences on intestinal motility and visceral hypersensitivity, however the mechanisms involved in these changes remain to be identified.
We used the neonatal-maternal separation (NMS) model as a psychological stressor, and studied glial-specific transcriptional signatures using Sox10CreERT2;RiboTag mice. Distal colons were collected at 16-20 weeks of age and RNA-seq of myenteric glial mRNA was performed using STAR and DESeq2. Specific transcripts of interest were localized in situ by RNAscope with co-immunolabeling to quantify cellular expression. Integrative physiological consequences were assessed by measuring visceral hypersensitivity and in vivo motility, and cellular physiological responses were studied using calcium imaging in Wnt1Cre;GCaMP5g-tdT/GfaphM3Dq mice, which express chemogenetic hM3Dq receptors in GFAP+ cells and GCaMP5g in neurons and glia.
In normal animals, more than 600 genes are differentially expressed between male and female enteric glia. Males that underwent NMS exhibited large shifts in glial genomic profiles with 469 genes upregulated, while subtler effects were observed in females with 124 genes downregulated. Interestingly, the gene expression patterns of male glia become more like those from females following NMS. In addition to the change in expression level, spatial distribution of target RNA, such as immune-related signaling gene Oasl2 (p<0.01) or Ifit1 (p<0.05), is affected by NMS. This remodeling of molecular architecture is associated with a higher excitability of enteric glia, demonstrated by an increased frequency of calcium responses after specific glial stimulation. Finally, we observed an increased visceral sensitivity in males after NMS (p<0.01), and a slower intestinal transit after glial specific activation in both sexes (p<0.05), which may be linked to the hyperexcitability of glia.
Together, these data show that early life adversity shifts the molecular architecture of enteric glia in a sex-specific manner, with a prominent effect in males where NMS promotes a ‘feminization’ of genomic signatures. This remodeling is associated with physiological consequences on intestinal motility and visceral hypersensitivity, however the mechanisms involved in these changes remain to be identified.
Presenter
Speakers
Tracks
Related Products
INTRODUCTION
SOCIETY: AGA
MONITORING OF CALPROTECTIN IN INFLAMMATORY BOWEL DISEASE USING A SWEAT BASED WEARABLE DEVICE
Switching from originator to biosimilar infliximab (IFX) is effective and safe. However, data on multiple switching are scarce. The Edinburgh IBD unit has undertaken three switch programmes: (1) Remicade to CT-P13 (2016), (2) CT-P13 to SB2 (2020), and (3) SB2 to CT-P13 (2021)…
AGA Novel Mechanisms of Intercellular Communication That Control Gut Motility?
This session will focus on intercellular signaling mechanisms between neurons and non-neuronal cells that control gut motor functions…
GLIAL CONNEXIN-43 HEMICHANNELS ARE CRITICAL DETERMINANTS OF GUT INFLAMMATION IN MOUSE POSTOPERATIVE ILEUS AND REACTIVE HUMAN ENTERIC GLIA
BACKGROUND: Perianal fistulae are highly morbid complications of Crohn’s disease (CD) with a cumulative incidence of 20-25%; despite demonstrated efficacy of infliximab treatment, estimates of complete resolution are only 20-30%. Ultimately, 20% of patients will require proctectomy…


