Society: AGA
Background: Postoperative ileus (POI) is a common consequence of abdominal surgery and a neuroinflammation-based disorder of the gastrointestinal tract, which results in motility disturbances compromising patients' recovery. The underlying neuroinflammation involves the activation of resident macrophages and monocyte-derived macrophages (MDM) infiltration into the intestine's muscularis externa (ME). Immune checkpoint proteins, including PD-1 and its ligands PD-L1 and PD-L2, play a prominent role in cancer immunology, but their role in non-oncological inflammation-driven diseases is still elusive. Herein, we studied the role of PD-1/PD-L1/PD-L2 signaling in POI.
Materials and methods: POI was induced by a surgical intestinal manipulation in wildtype, PD-1-/-, PD-L1-/- and PD-L2-/- mice. We performed flow cytometry to identify the cellular sources of PD-1 and PD-L1 and used a blood cell transfer model to determine the role of a leukocyte-restricted PD-1 deficiency. qPCR and SMART-Seq2 analyses were used to analyze transcriptomes of sorted CX3CR1GFP MDM from wildtype and PD-1-/- macrophage populations. Immunofluorescence microscopy visualized neuronal cell loss, PD-1 and PD-L2 expression and in vivo macrophage phagocytosis assay and GI transit measurements were used as functional readouts of PD-1 deficiency.
Results: PD-1, PD-L1 and PD-L2 mRNA levels were induced in the postoperative ME upon intestinal manipulation. PD-1-/- and PD-L1-/- but not PD-L2-/- mice were protected from POI- PD-1-/- mice showed a 50% reduction of MDM infiltration and reduced inflammation-mediated neuronal loss. Ly6C-Cx3CR1+ resident macrophages and Ly6C+Cx3CR1+ MDM were identified as the primary cellular source of PD-1. Both cell populations showed reduced in vivo phagocytosis under PD-1 deficiency, and a cell transfer experiment revealed a more than 60% reduced postoperative ME infiltration of PD-1-deficient compared to wildtype controls. The primary PD-L1 source are infiltrating MDM, monocytes and neutrophils. Gene ontology (GO) analysis within the ME of PD-1-/- mice revealed lower enrichment levels of genes regulating metabolic respiratory chain processes, oxidative stress, and immune functions. Moreover, sorted CX3CR1+Ly6C+ MDMs of PD-1-/- mice showed stronger enrichment of genes associated with various immune functions, host defense and negative regulation of immune and neuronal cell death.
Conclusion: Our data provide new evidence on the role of PD-1 signaling in MDM during postoperative intestinal neuroinflammation following abdominal surgery. PD-1 deficiency prevents POI, results in a metabolic switch, and reduces infiltration capacity and phagocytosis of MDM. We conclude that interaction in PD-1 signaling might be a potential target in prevetion of POI or other immune-driven intestinal disorders.
Pain is a cardinal sign of inflammation and is associated with flares in Inflammatory Bowel Disease (IBD) patients. It is commonly accepted that inflammatory mediators released by inflamed tissues and infiltrated inflammatory cells are responsible for activation of peripheral nociceptors and activation of pain pathways. However, 30% to 50% of IBD patients in remission still report significant pain, despite the absence of infiltrated inflammatory cells, complete repair of tissues and restoration of normal bowel habits. We hypothesized that histologically repaired tissues from patients in remission are releasing mediators that signal to sensory neurons and contribute to chronic pain symptoms. We aimed at understanding whether peripheral mediators present in colonic tissues of IBD patients in remission could contribute to the sensitization of peripheral nociceptors, thereby potentially activating pain pathways, and we investigated a potential role for Protease-Activated Receptor-1 (PAR1). Methods. IBD patients in remission or healthy control (HC) individuals (colon cancer screening presenting no pathology) had colonoscopy at the Gastroenterology Clinic of the Toulouse Hospital. Colon biopsies were harvested and freshly incubated in culture media for 1h. The obtained culture media were exposed to dorsal root ganglia (DRG) neurons primary cultures (either mouse DRG cultures or human DRG cultures obtained from brain-dead organ-donor patients at the Montpellier Hospital). Calcium signals in DRG neurons were recorded after their exposure to HC or IBD patient biopsy supernatants, in the presence or not of a PAR1 antagonist. Further, the effects of an oral treatment with a PAR1 antagonist (CVT120165) in a rat model of colitis induced by the intracolonic administration of trinitrobenzene sulfonic acid (TNBS) were investigated on abdominal nociceptive response to von Frey filament application and on tissue repair by histology scoring. Results. Colon biopsy supernatants from IBD patients in remission caused a significant increase in DRG neuron activation (both with mouse p<0.001, and human DRG neurons p<0.01), compared to the effects of HC biopsy supernatants. Pre-incubation of DRG neurons (both mouse or humans) with a PAR1 antagonist significantly inhibited IBD supernatants-induced DRG calcium signals. Oral treatment with the PAR1 antagonist CVT120165 significantly inhibited, in a dose-dependent manner, referred abdominal pain in rats 7-days after the induction of TNBS colitis, and also favored tissue repair. Conclusions. Peripheral mediators in the colon of IBD patients in remission activate sensory neurons by a PAR1-mediated mechanism. PAR1 blockade alleviates from pain symptoms in a rat model of colitis and helps tissue repair. Altogether, PAR1 appears as a good target for the treatment of IBD-associated pain in remission.
Background: The current prevalent paradigm focuses on small intestinal (SI) bacterial overgrowth (SIBO) as a cause of gastrointestinal (GI) symptoms, such as abdominal pain. However, recent studies have found that alterations in SI microbial composition rather than SIBO may underlie GI symptoms commonly seen in patients with disorders of gut-brain axis interaction. Human stool is commonly used to recapitulate the human gut microbiome in germ free (GF) mice. Stool contains bacteria from the entire GI tract, and while stool faithfully captures colonic bacteria, it remains unclear if stool can also recapitulate the SI microbiome in mice.
Aim: To establish a mouse model that replicates the human SI microbiome and investigate the influence of the human SI microbiome on visceral sensitivity.
Methods: Duodenal aspirate and stool samples were collected from healthy controls (HC) and age/sex matched patients with abdominal pain (SIBO and bacterial pathogen culture negative). GF mice were gavaged with human SI aspirate or stool and were maintained in gnotobiotic isolators or ISOcage™ system. After 4 weeks, SI contents from mice were collected and processed with the original human input samples (SI aspirate or stool) for 16S rRNA sequencing. In a subset of mice, visceromotor response (VMR) to colorectal balloon distension (CRD) was measured during 10-second distension intervals of 15, 30, 45 and 60 mmHg using a solid-state manometry catheter in the colon.
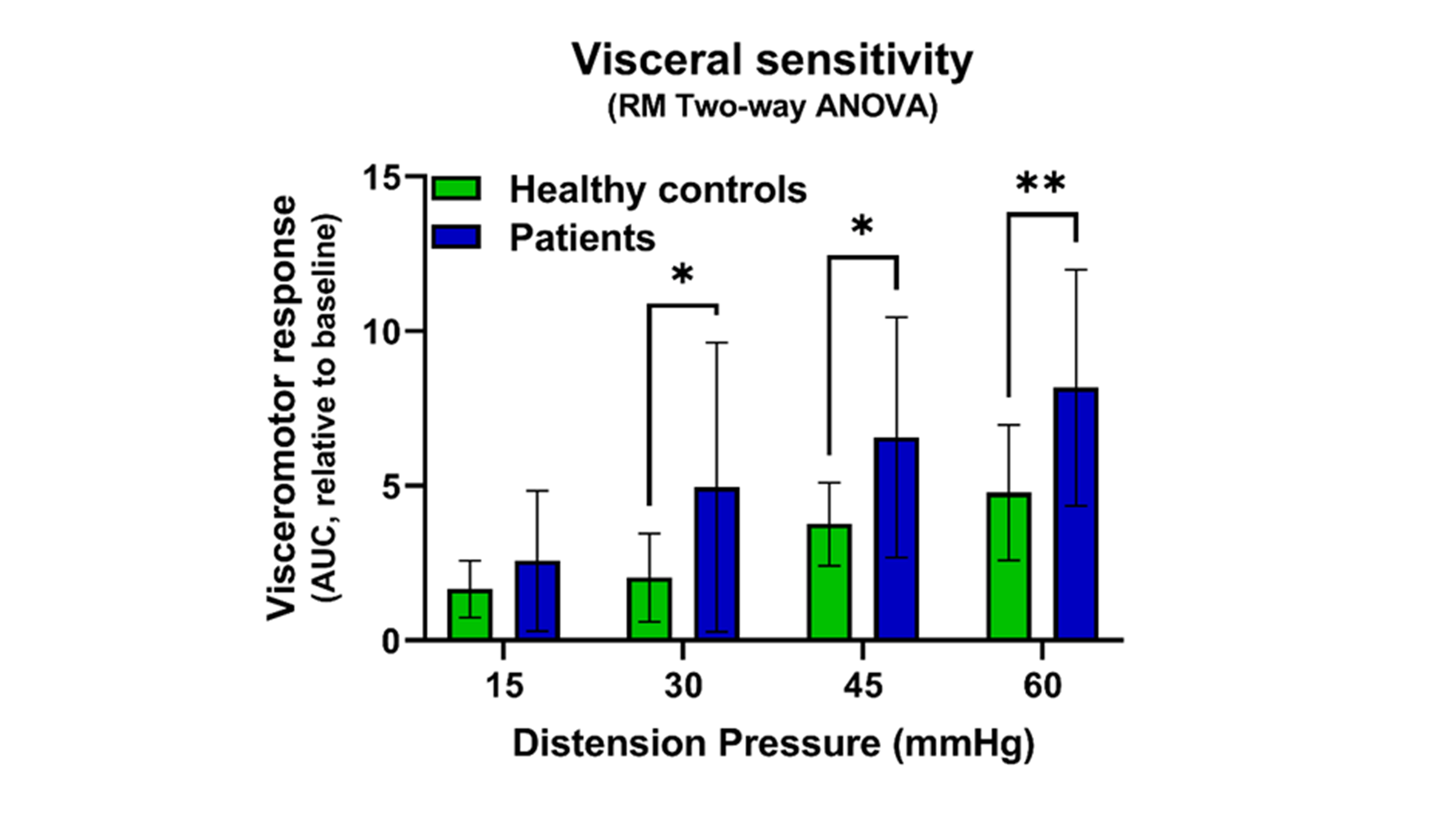
Results: To determine the input sample that best recapitulates the human SI microbiome, we compared the mouse and human SI microbiome following colonization of GF mice with human SI contents or stool (n=3 donors, 3-4 mice/donor). β-diversity-based principal coordinate analysis showed that the SI microbiome of mice, colonized with human SI aspirate, more closely resembled the human input sample (80% of human SI species were present in mouse SI) than the SI microbiome of mice, colonized with human stool (40% of species were retrieved) (Fig. 1). Hence, we colonized GF mice (4-12 mice/donor, 50% males) with SI contents from HC (n=3) or patients with abdominal pain (n=2) to study the effect of SI microbiome on GI physiology. Post-colonization, mice with SI contents from patients had significantly higher VMR to CRD when compared to mice colonized with SI aspirates from HC (Fig. 2). We sequenced human and mouse SI contents from 1 abdominal pain patient and 2 HCs and found a Shigella spp. in the human and mouse SI content from the patient. The relative abundance of Shigella spp. in the mouse SI positively correlated with the VMR to CRD (ρ=0.86, p<0.001).
Conclusion: Human SI contents are better than stool for replicating the human SI microbiome in mice. The presence of Shigella spp. in the SI may underlie visceral hypersensitivity in patients with abdominal pain and represents an important therapeutic target.
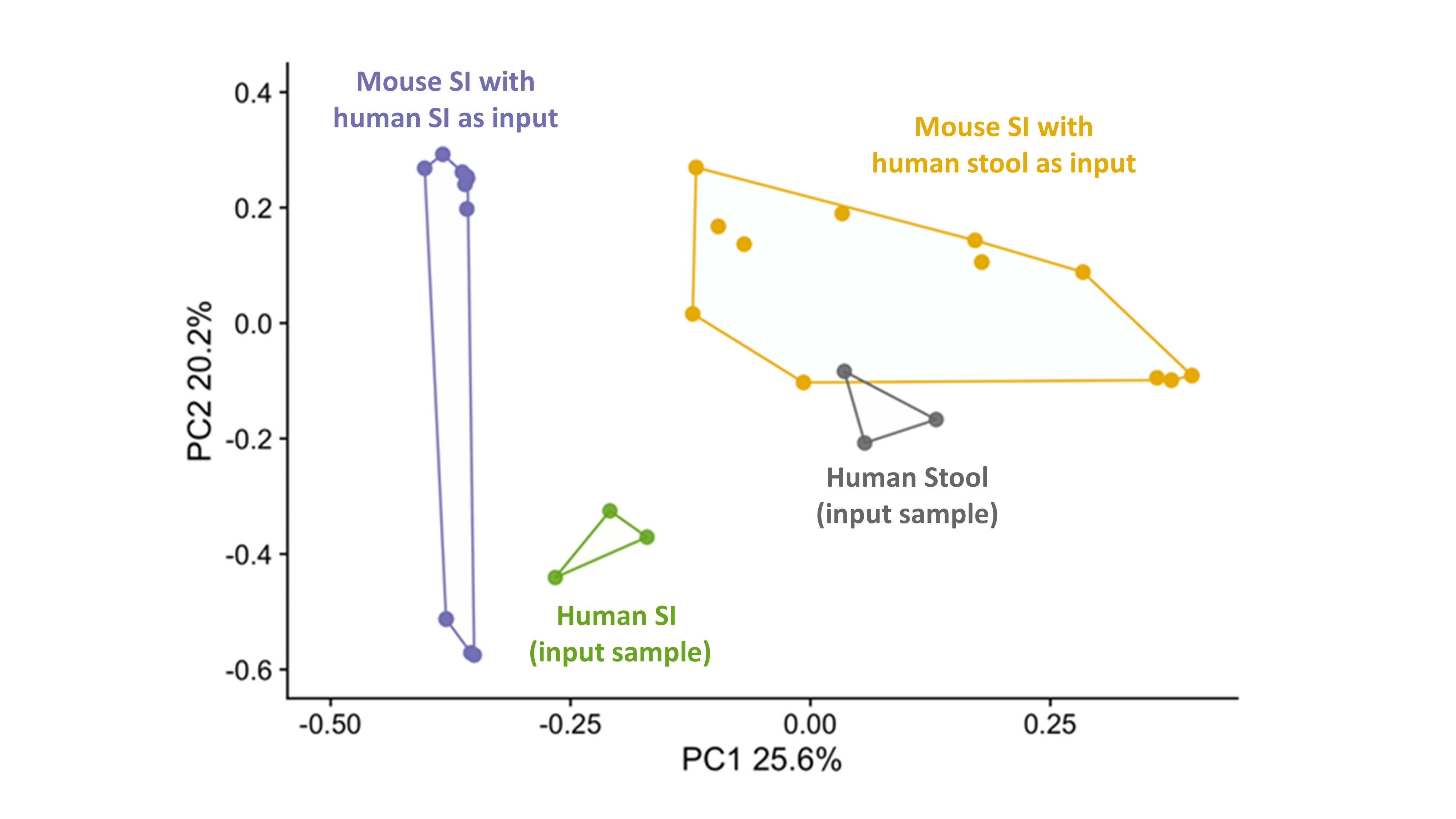
Figure 1. Beta diversity-based principal coordinate analysis of SI contents of mice gavaged with human SI aspirates (purple) and human stool (yellow) and the original human input sample (human SI, green and human stool, grey).
Figure 2. SI dysbiosis induces visceral hypersensitivity. The visceromotor response of mice, gavaged with SI aspirates from patients with abdominal pain, was increased at distension pressures of 30, 45 and 60 mmHg when compared to mice, gavaged with SI aspirates from healthy controls. Repeated measurements Two-Way ANOVA with Bonferroni post-hoc correction to account for multiple mice colonized with the same donor sample: *p<0.05, **p<0.01.
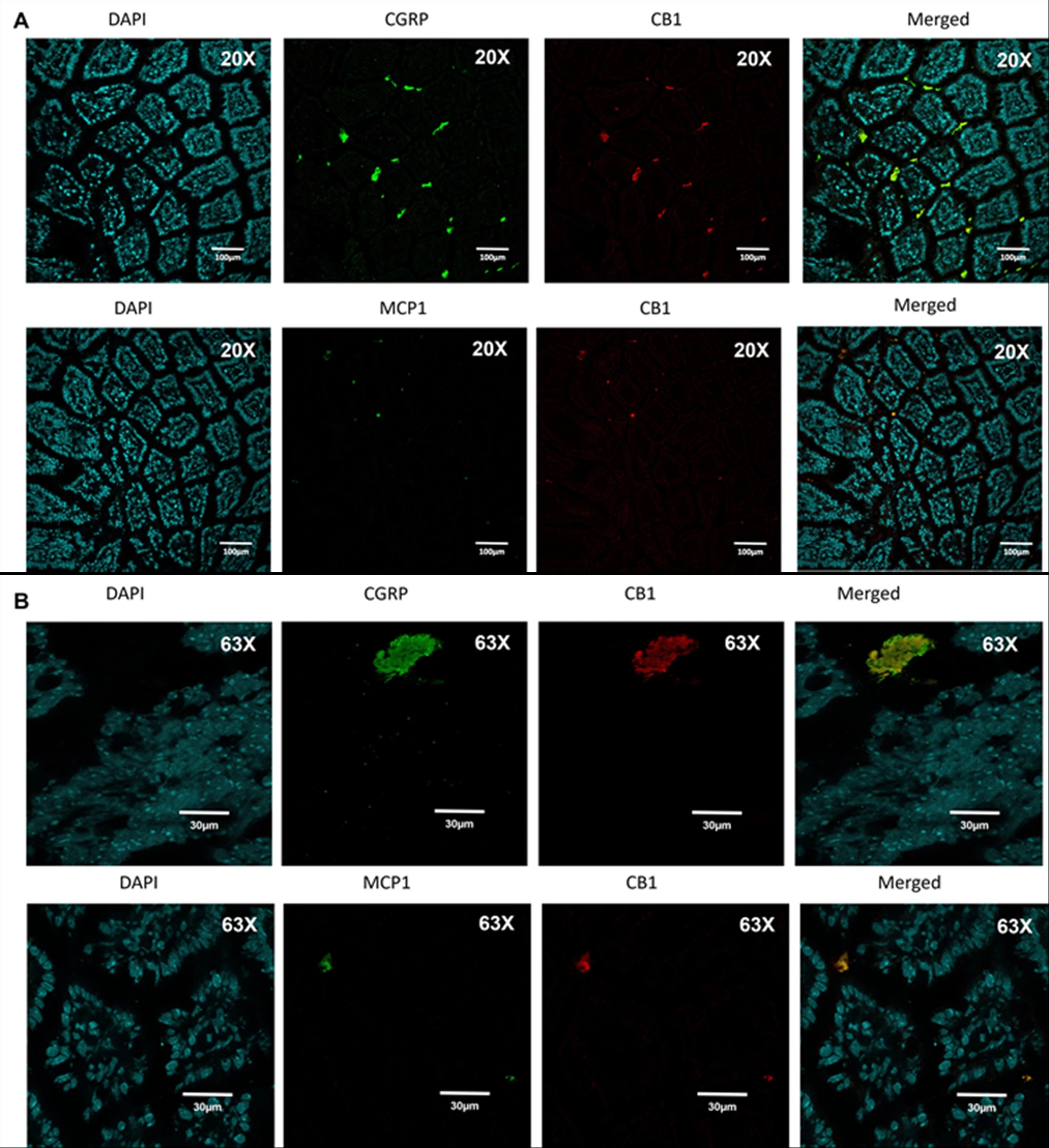
Background: We recently showed that splanchnic nerve plays a key role in normal physiology of energy regulation and post-gastric bypass (RYGB), where its activity is significantly increased to induce “browning” of mesenteric fat. Administration of an endocannabinoid receptor-1 (CB1) inverse agonist (Rimonabant) increased splanchnic nerve activity and replicated an energy balance phenotype in mice that similar to that induced by RYGB. We also showed that splanchnic denervation significantly reduces the response to Rimonabant. Methods: We explored : 1) where these peripheral CB1 are located in the gut and how do they mediate their energy regulatory effects using surgical mouse models. 2) whether gut microbiome changes post-gastric bypass mediate the energy effect of the surgery through the CB1-expressing cells. Results: We found that intestinal CB1 co-localizes mainly with CGRP-expressing cells (sensory neurons) and not epithelial, enteroendocrine or immune cells (Figure 1). We then developed and confirmed a model of afferent selective denervation (aSpDNV) using topical capsaicin over celiac ganglia and its branches. Interestingly, aSpDNV mice express a phase of increase food intake but no change in body weight. Furthermore, fecal microbial transfer from RYGB and Sham -operated mice into naïf recipients induces a significant increase in afferent and efferent splanchnic nerve activity of RYGB-stool recipients compared to their controls (Figure 2). Finally, intestinal CB1 expression was significantly lower in RYGB-stool recipients compared to their controls. Conclusion: Gut microbiome can communicate energy signals directly to the brain through CB1-expressing sensory neurons.
BACKGROUND: Modern techniques allow specific target of glial subtypes to examine their roles in visceral hypersensitivity and pain. Satellite glial cells (SGCs) intimately interact with sensory neurons in dorsal root ganglia (DRG), however, their specific roles in regulating DRG neuron activity in intact animals in influencing colonic sensitivity and pain behaviors are not characterized. AIMS: (1) To examine the effects of cell-targeted activation of SGCs on sensory neuron activity/excitability, and (2) to characterize the functional role of SGCs in regulation of colonic mechanosensitivity and pain. METHODS: Using cre-loxP technique to specifically activate SGCs by expressing hM3Dq in proteolipid protein (PLP)- or glial fibrillary acidic protein (GFAP)-expressing cells followed by clozapine N-oxide (CNO, 1-2 mg/kg intraperitoneally (i.p.) or 26 µM intrathecally (i.t.)) treatment. The number of hM3Dq-expressing SGCs is measured by flow cytometry. The activity/excitability of mechanosensory neurons were assessed by electroimaging responding to glass pipette poking or shear stress along with D-GsMtx4, a PIEZO2 inhibitor (10 µM). Colonic mechanosensing was measured by colonometry. Pain behaviors were assessed by hindpaw withdrawal responses to Von-Frey stimulation, hot plate, and gait assay(s). Calcitonin gene-related peptide (CGRP) expression was examined by immunohistochemistry/slot blot. Neurogenic inflammation was assessed by H&E stain. RESULTS: Chemogenetic activation of SGCs (i.p.) induced colonic hypersensitivity and somatic mechanical pain in male but not female mice (n>3; p<0.05), with no change in thermal sensitivity in either sex. hM3Dq expression was strongly present in SGCs of DRG with higher levels in male than female mice (p<0.05), some in hindpaw, and none in the colon or spinal cord of both sexes. Activation of SGCs also increased CGRP levels in DRG neurons (3.5-fold, p<0.05), adjacent to activated SGCs, and in spinal dorsal horn extending to the central commissure in male mice but not female mice, enhanced the activity/excitability of mechanosensory DRG neurons (38/55 (69%) vs 9/23 (39% in control) responding to poking; 260/330 neurons (79%) vs 77/280 (28% in control) responding to shear), and induced neurogenic inflammation in the hindpaw and colon. Results from i.t. CNO-treated Plp;hM3Dq mice and i.p. CNO-treated GFAP;hM3Dq mice recapitulated the behavioral and molecular results in i.p. CNO-treated PLP;hM3Dq mice. CONCLUSION: The augmentation in colonic mechanical sensitivity, somatic mechanical pain, CGRP expression in DRG neurons in male mice but not female mice following SGC activation suggest a sexual dimorphic role of SGCs in regulation of visceral hypersensitivity and pain. Elevation in the activity/excitability of mechanosensory neurons after SGC activation reveal an SGC-mechanosensory neuron crosstalk in pain processing.