274 - AUTOLOGOUS STEM CELL TRANSPLANT MAY “RESET” NOT ONLY THE IMMUNE SYSTEM BUT MICROBIOME STRUCTURE AND FUNCTION IN PATIENTS WITH CROHN’S DISEASE
The microbiome plays a central role in intestinal inflammation in IBD. It is influenced by environmental factors included diet. In turn, the microbiome can incite intestinal inflammation
Methods This study includes two independent cohorts comprised of Crohn’s disease (CD) and healthy controls, respectively Hong Kong (92 CD, 21 controls) and Australian (98 CD, 21 controls). Previous and current consumption of P80 was quantified using a validated 26-item food additive questionnaire. Fecal microbiota composition was assessed by shotgun metagenomic sequencing. The interaction between P80 and P. mirabilis was investigated in both in vitro and in vivo models. The effect of P80 on P. mirabilis was determined by bacterial transcriptome sequencing. The interested protein was identified by silver straining followed by mass spectrometry.
Results P. mirabilis was identified as the enriched microbiota in the CD patients with high food additive consumption when compared to controls in both Hong Kong and Australia cohorts. Co-cultivation of P. mirabilis and P80 induced more cell death on human epithelial cells lines, INT 407 and NCM 460, compared to P. mirabilis alone or Escherichia coli control groups (Figure 1A). P80 exposure increased expression of virulence genes in P. mirabilis by RNA-seq. The co-cultured conditional medium of P. mirabilis and P80 (Plus.CM) triggered cell death, which was attenuated by Proteinase K, implying that the secreted protein is the functional participant in this process (Figure 1B). Compared to other fractions, the proteins with molecular weight over 100kDa exhibited the most notable function. By proteomics identification, P. mirabilis-derived enzyme X was uncovered as the potential deleterious protein in arising and aggravating inflammation (Figure 1C). In mouse models, the mice orally challenged with P. mirabilis and fed with P80 diet had shortened colon length (Figure 2A) and higher Lipocalin-2 (LCN2) levels (Figure 2B) compared to P. mirabilis solely and control groups.
Conclusion We have explored that with the addition of P80, P. mirabilis exacerbated the inflammation both in vitro and in vivo. The enzyme X, derived from P. mirabilis, was identified being the possible deleterious participant in this process and may be targeted for future precision therapy.
This work is supported by the Croucher Senior Research Fellowship and The Leona M. and Harry B. Helmsley Charitable Trust.
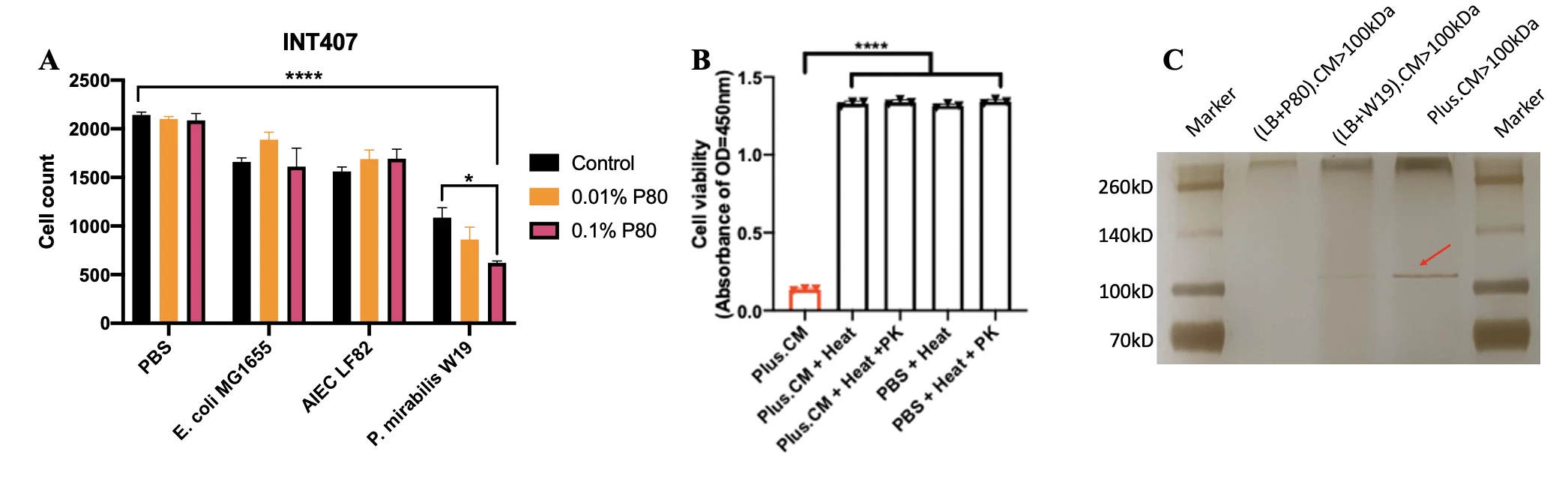
Figure 1. (A) Exposure to both P. mirabilis and P80 induced more cell death, in comparison with P. mirabilis alone or other control groups. (B) The Proteinase K treatment attenuated the function of co-cultured conditional medium in triggering cell death. (C) Silver staining of protein fractions above 100kDa indicated the targeted enzyme. Statistical significance was determined by 1-way or 2-way analysis of variance appropriately. ****P < .0001.
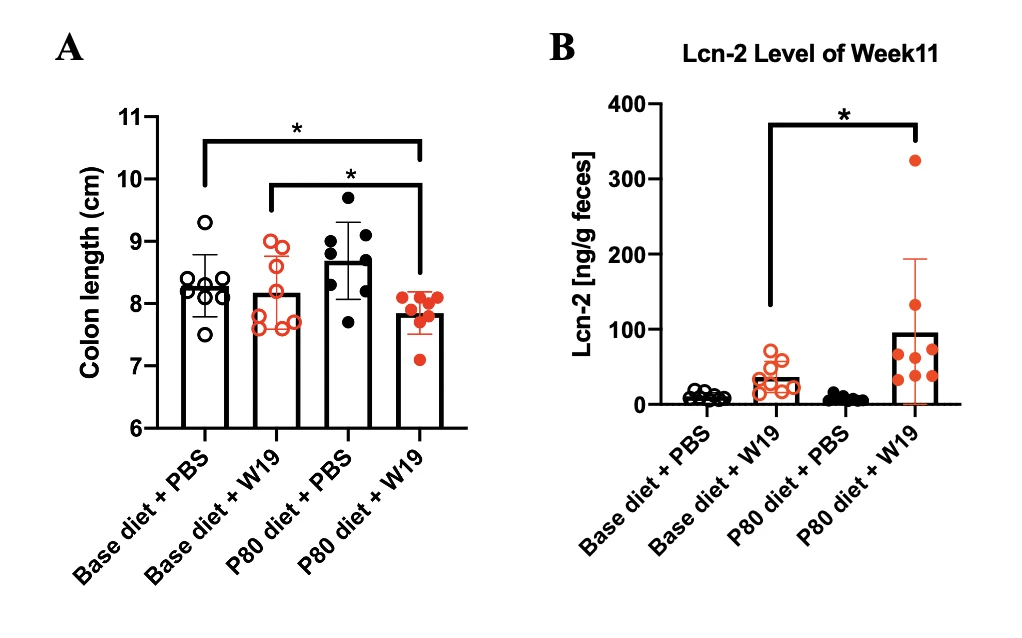
Figure 2. Mice challenged with P. mirabilis and P80 diet (A) shortened colon length and (B) induced higher level of Lcn-2 expression. Statistical significance was determined by 1-way or 2-way analysis of variance appropriately. *P < .05.
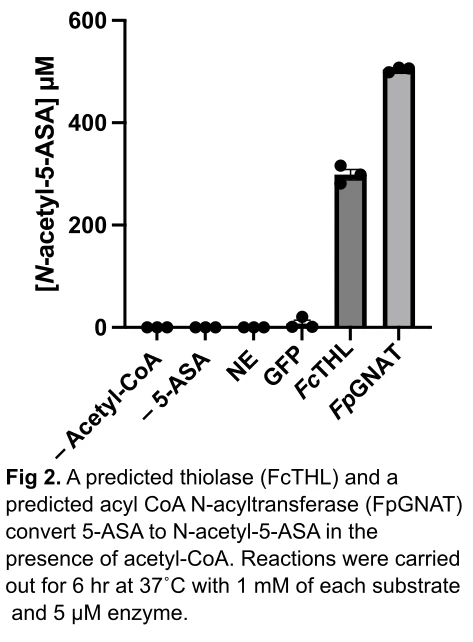
Methods: We examined the association between gut microbial acetyltransferases with risk of 5-ASA treatment failure in the “Study of a Prospective Adult Research Cohort with IBD” (SPARC IBD). We included 208 participants who at baseline 1) gave a stool sample, 2) were on 5-ASA, and 3) were steroid-free. Fecal metagenomic data was processed by biobakery3. Exposure was defined as metagenomic carriage of 3-4 drug-degrading acetyltransferases compared to 0-2 acetyltransferases, as in the IBDMDB. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) of incident steroid use via multivariable generalized estimating equations, adjusting for age and sex. For biochemical characterization, the 12 enzymes identified in the IBDMDB were heterologously expressed by E. coli. A representative enzyme was selected from each protein family for purification, and then incubated with 5-ASA and acetyl CoA for 6 hr at 37C. Using a custom LC–MS assay, we detected N-acetyl 5-ASA production. Finally, to gain mechanistic insight into how these enzymes convert 5-ASA, we made efforts to crystallize the two purified proteins.
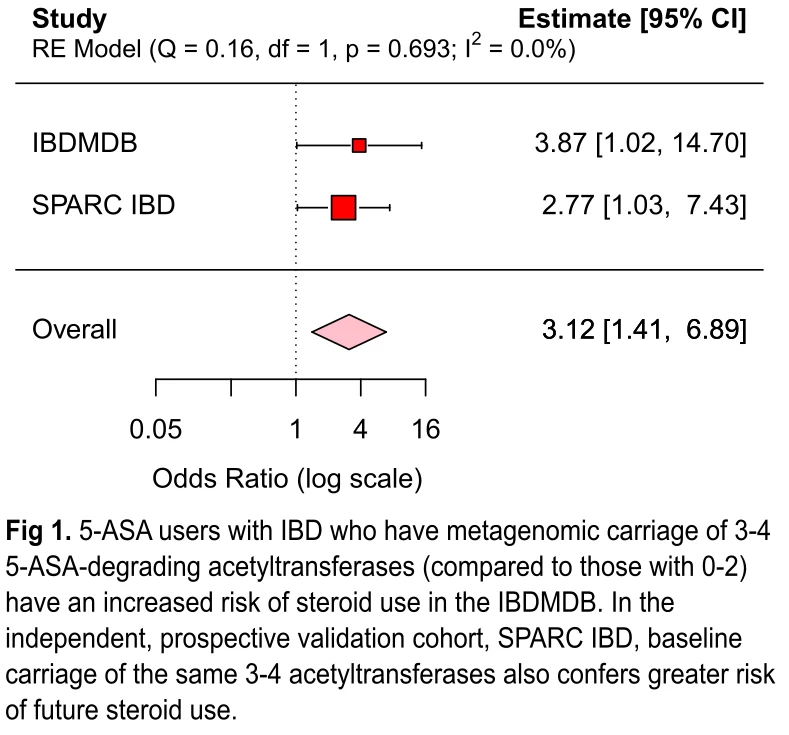
Results: Over a median follow-up of 8 months, we identified 60 cases of corticosteroid use. Consistent with the IBDMDB, we found that metagenomic carriage of 3 or more microbial acetyltransferase genes in SPARC IBD (compared to 2 or fewer) was associated with treatment failure (OR 2.77, 95% CI 1.03-7.43). In a meta-analysis of the two cohorts, we observed a three-fold increased risk of drug failure (OR 3.12, 95% CI 1.41-6.89) (Fig 1). In vitro experiments confirmed the ability of a thiolase and an acyl-CoA N-acetyltransferase to acetylate 5-ASA, with >25% conversion (Fig 2). Finally, we generated an acetylated unliganded crystal structure of the thiolase (1.9Å), and found a catalytic cysteine residue poised to acetylate an incoming substrate.
Conclusions: We characterized two gut microbial protein families that are directly involved in 5-ASA metabolism and, in turn, are prospectively associated with 5-ASA treatment failure. These findings advance the possibility of microbiome-based personalized medicine for patients with IBD.
For patients with refractory Crohn’s disease (CD) autologous stem cell transplant (auto-SCT) can induce disease remission in the majority of patients.1,2 The therapeutic mechanism of auto-SCT is unknown though it is believed to induce an immune “reset”. The impact of auto-SCT on the CD microbiome composition and function has never been studied.
Methods
14 patients with CD were enrolled in a Phase IIa study (NCT03219359). Blood, intestine and stool samples were taken prior to transplant and at intervals post-transplant. Fresh leukocytes were isolated and analyzed by mass cytometry (CyTOF). Microbiome composition was analyzed by 16S sequencing. Patient fecal slurries were inoculated by oral gavage into 6 wk gnotobiotic C57BL/6 mice (M/F). At 4 wks, mouse intestinal leukocytes were analyzed by flow cytometry (Aurora).
Results
Microbial community analysis revealed a significant decrease in diversity post-transplant at hospital discharge followed by a restoration of diversity to baseline by 3 months post-transplant. (Fig 1A) Principal component analysis failed to resolve population differences at any time point during auto-SCT. (Fig 1B) While microbial population statistics did not differ 6 months post-transplant, comparison of microbial composition suggested several changes.(Fig 1C) To understand the magnitude of microbial compositional changes we compared CD patients who underwent auto-SCT to patients treated with ustekinumab.3 This analysis suggested a much more significant change in species composition post auto-SCT compared to treatment with ustekinumab. (Fig 1D) The significant decrease in diversity at discharge suggests long-term changes in microbial composition may be facilitated by damage to microbiome structure during transplant.4 Comparing microbial composition at discharge to 6 months confirms new organisms are acquired after discharge.(Fig 1E) Analysis of blood and intestinal immune populations post auto-SCT indicates the most significant changes occur among intestinal CD14+ populations.(Fig 2A) Using gnotobiotic mice we studied whether microbial functions post auto-SCT may reinforce changes in immune cell populations. In one patient where there was an increase in intestinal CD14+ populations post-transplant we observed a similar increase in Ly6C+ populations in mice colonized with stool taken at 6 months post-transplant compared to colonization with stool taken at baseline (Fig 2B, pt CD021). We did not observe a similar increase in mice colonized with patient stool where there was no increase in CD14+ populations (Fig 2B, pt CD015).
Conclusion
Our initial analysis of microbiome function and diversity during auto-SCT suggests this treatment modality creates a uniquely fertile ground where microbial composition can be “reset” and the function of these new microbes may reinforce changes in the mucosal immune system.
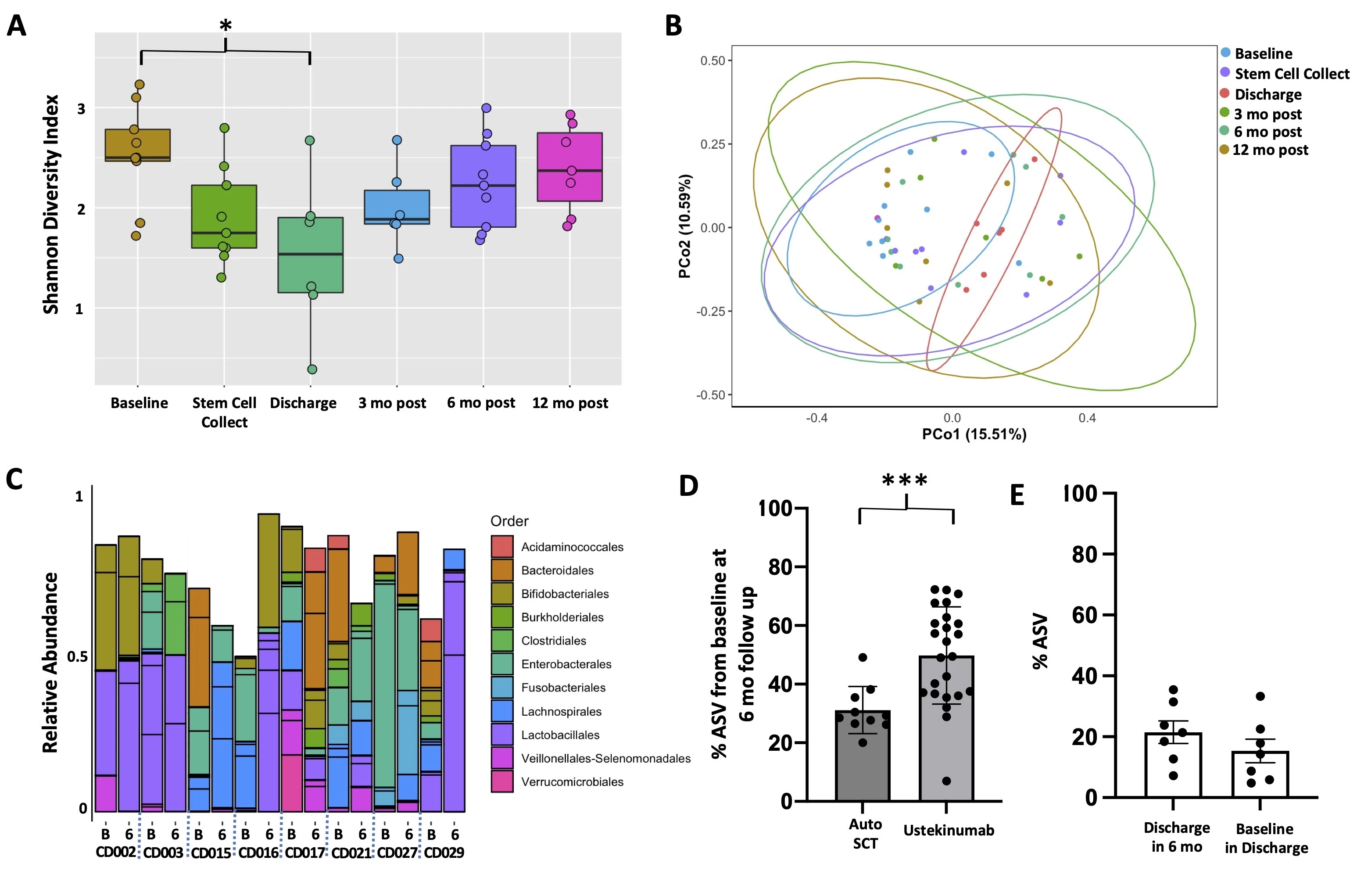
Fig 1. Bacterial DNA was extracted and the V4 region of the 16S rRNA gene amplified and sequenced (Illumina MiSeq). Amplicon sequence variants (ASV) were assigned and classified using DADA2. A, Mean (95% CI) Shannon diversity indices are plotted. Comparison by ANOVA with Tukey post hoc. B, Principal component analysis. C, Relative abundance of bacterial composition (order) at baseline (B) and 6 months post-transplant (6). Patients ID CDXXX. D, ASV were compared at baseline to follow up in patients undergoing auto-SCT or treatment with ustekinumab. Percent of ASV present at baseline and found in follow up relative to the total number of ASV at follow up are plotted (mean +/- SEM). Samples are compared by Mann Whitney test (p = 0.0005). E, Percent of ASV overlap at different timepoints are plotted demonstrating that the ASV present at discharge post stem cell transplant are not derived from baseline microbiota nor do they influence the post-transplant populations.
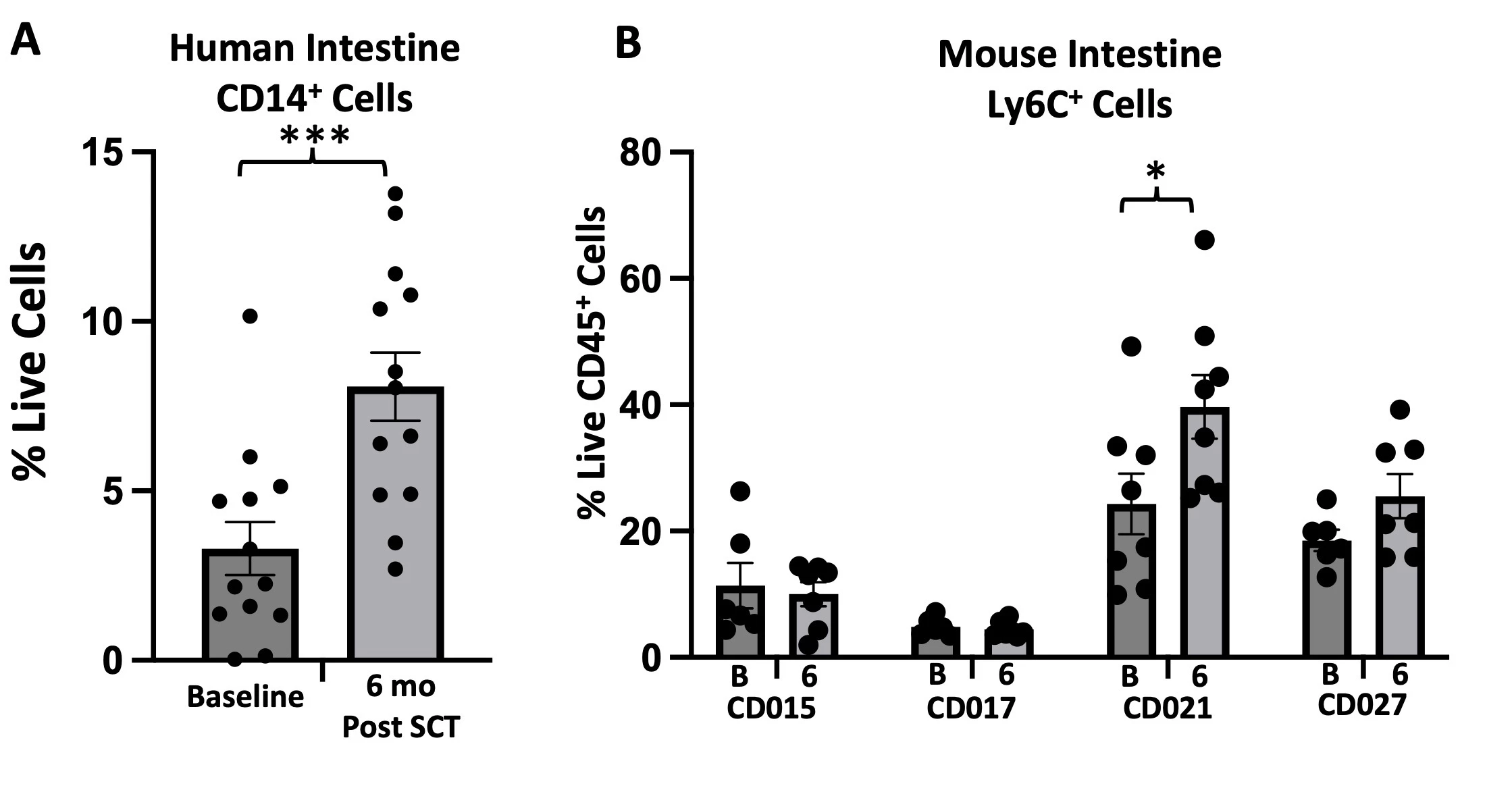
Fig 2. A, Human intestinal leukocytes are analyzed by CyTOF. CD14+CD3-CD19-HLADR+ cells are plotted as a percent of live singlets (mean +/- SEM). Samples compared by Mann Whitney test (p = 0.0006). B, 6 week germ free mice are colonized with stool samples from 4 patients (8 mice per group; 4M, 4F). Mice are colonized with stools from baseline (B) or 6 month follow up (6). Intestinal leukocytes are isolated and analyzed by flow cytometry (Aurora). B220-MHCII-Ly6C+ cells are plotted as a percent of live CD45+ cells. Samples are compared by Mann Whitney test (p = 0.04 for CD021).


